
Magic numbersĪnother important point is that the rules governing the shell-filling are different for nucleons from those for electrons. In addition, elements with even numbers of protons tend to have many stable isotopes, while the odd-number elements have few stable isotopes. In fact, of the 273 stable nuclei, only four have odd numbers of both neutrons and protons. In “normal” chemistry, electron-pairing is important, but for nuclei, proton-pairing and neutron-pairing within the nucleus is even more important. That is, we can consider protons and neutrons filling shells just like we do electrons. Stable element isotopes reflect two phenomena both related to the nuclear shell model of the atom. The preference for even numbers of nucleons One can use a simple model that increasing quantities of neutron “glue” are needed to hold together the positively-charged protons. The reason is that the proton:neutron ratio in stable (and long-lived radioactive) isotopes increases from about 1:1 for the early elements to 1:1.6 for uranium-238. Not only must the projectile have a high enough atomic number, but just as important, it must have as many neutrons as possible. The target element must have as high an atomic (proton) number as possible. The new elements are synthesized by firing beams of one element at a target of another element. 1999‑2023 - All Rights Reserved.When will the first element of Period 8 be synthesized? Which element will it be? Which country will first accomplish the task? Now that each element in Period 7 has been synthesized, these are the questions on the minds of every nuclear chemist and physicist. Retrieved from Ĭopyright © Israel Science and Technology Directory. "Sortable list of elements of the Periodic Table". The story behind the discovery that elements are born in stars.Atomic Weights of the Elements (From IUPAC).Multilingual Dictionary and Etymology of the Periodic Table Elements.Atomic Reference Data for Electronic Structure Calculations.List of Periodic Table Elements in Hebrew.Other resources related to the Periodic Table For these elements, the weight value represents the mass number of the longest-lived isotope of the element.Įlectron configuration: See next page for explanation of electron configuration of atoms. The elements marked with an asterisk have no stable nuclides. The values shown here are based on the IUPAC Commission determinations ( Pure Appl. For relative abundances of isotopes in nature, see reference on Atomic Weights and Isotopic Compositions.Ītomic weight: Atomic weight values represent weighted average of the masses of all naturally occurring isotopes of an element. The abundance of each isotope depends on the source of materials.
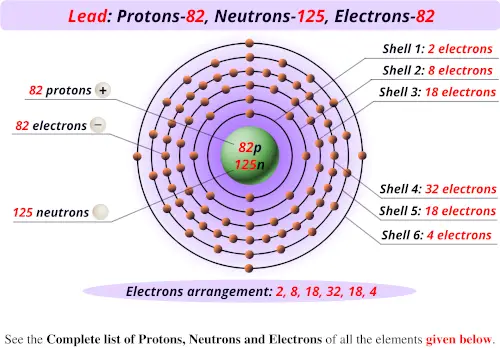
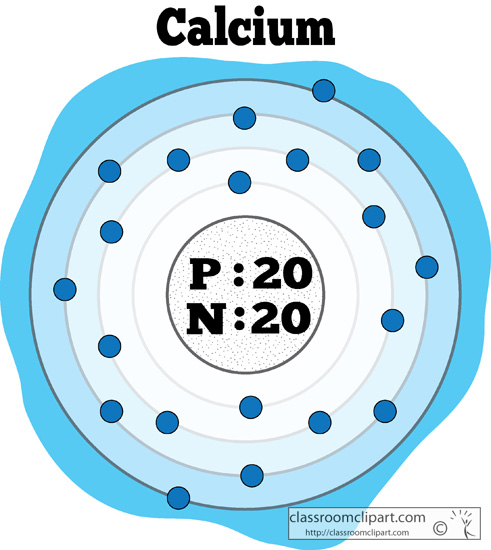
For example, the two common isotopes of carbon, 12C and 13C, have 6 and 7 neutrons, respectively. Elements have more than one isotope with varying numbers of neutrons. The isotope of an element is defined by the sum of the number of protons and neutrons in its nucleus. Isotope: Atoms of the same element with the same atomic number, but a different number of neutrons. Thus, each proton and neutron has a mass of about 1 amu. This isotope of carbon has 6 protons and 6 neutrons. Atomic mass is measured in Atomic Mass Units (amu), which are scaled relative to carbon, 12C, that is taken as a standard element with an atomic mass of 12. Each element is uniquely defined by its atomic number.Ītomic mass: The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus. Boiling pointĪtomic number: The number of protons in an atom. For the Year of Discovery of elements see the list with the English and Hebrew names.For these elements, the weight value shown represents the mass number of the longest-lived isotope of the element. The elements marked with an asterisk (in the 2nd column) have no stable nuclides.Lanthanoids and Actinoids are numbered as 101 and 102 to separate them in sorting by group. Group: There are only 18 groups in the periodic table that constitute the columns of the table.Elemental compositions of crustal rocks differ between different localities ( see article). Earth crust composition average values are from a report by F.In a sorted list, these elements are shown before other elements that have boiling points >0☌. The density of elements with boiling points below 0☌ is given in g/l.List of Periodic Table elements sorted by → Atomic number No.


 0 kommentar(er)
0 kommentar(er)
